Inorganic oxysalts are inorganic compounds that can be considered as salts of simple inorganic oxyacids. The structure of an inorganic oxysalt consists of anionic subunits interlinked by some additional charge-compensating agents. As a rule, description of the structure of an inorganic oxysalt is based upon subdivision in it of the strongest structural unit.
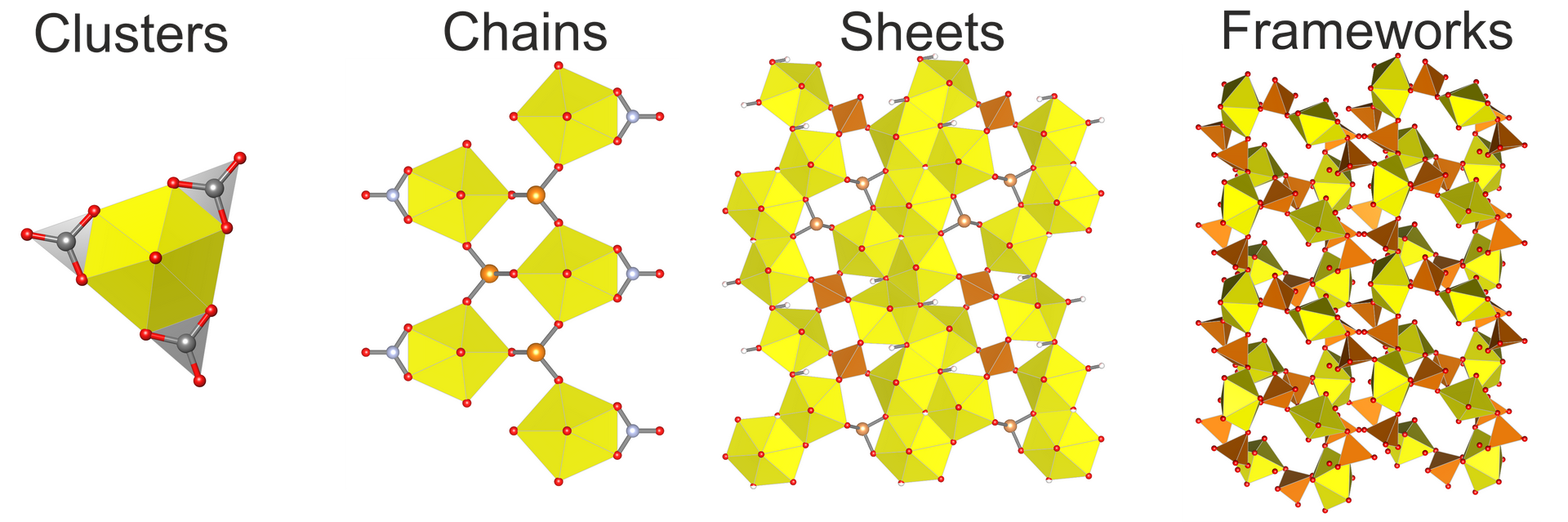
For a more detailed classification, an approach based on the dimension of the structural unit is used:
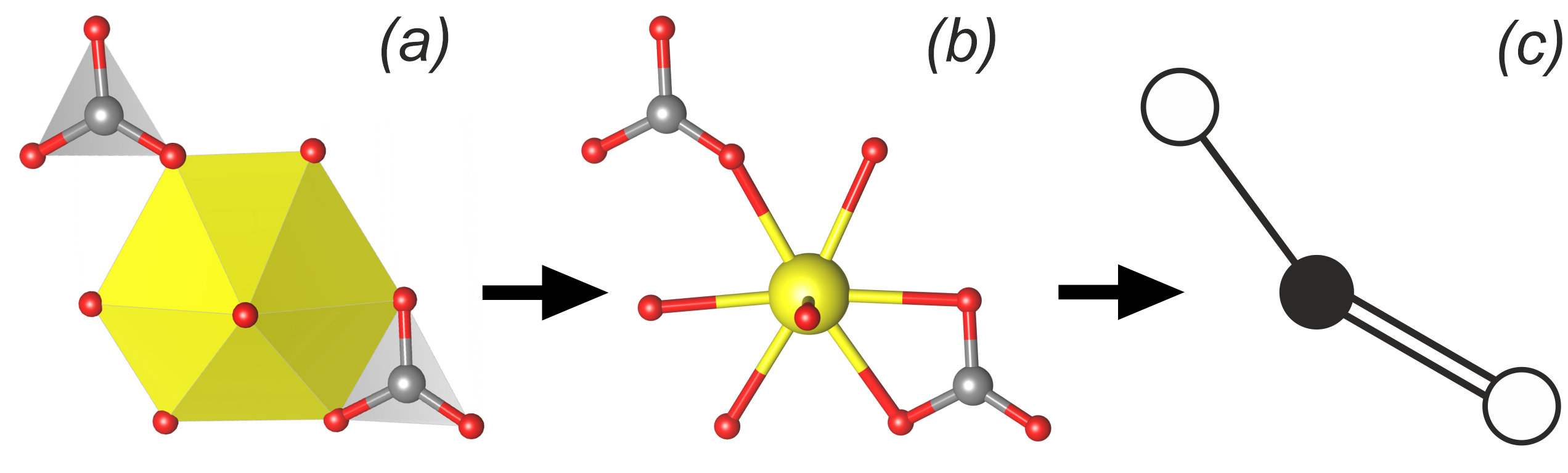
For a more detailed classification of uranyl structures, a method based on graph theory is used. If the structural complex includes only two types of polyhedra, then they can be designated as black and white vertices, respectively. Let two polyhedra have a common oxygen atom: then the vertices corresponding to them will be connected by an edge. Thus, one can compare to each structural (a,b) complex its two-color undirected graph (c).
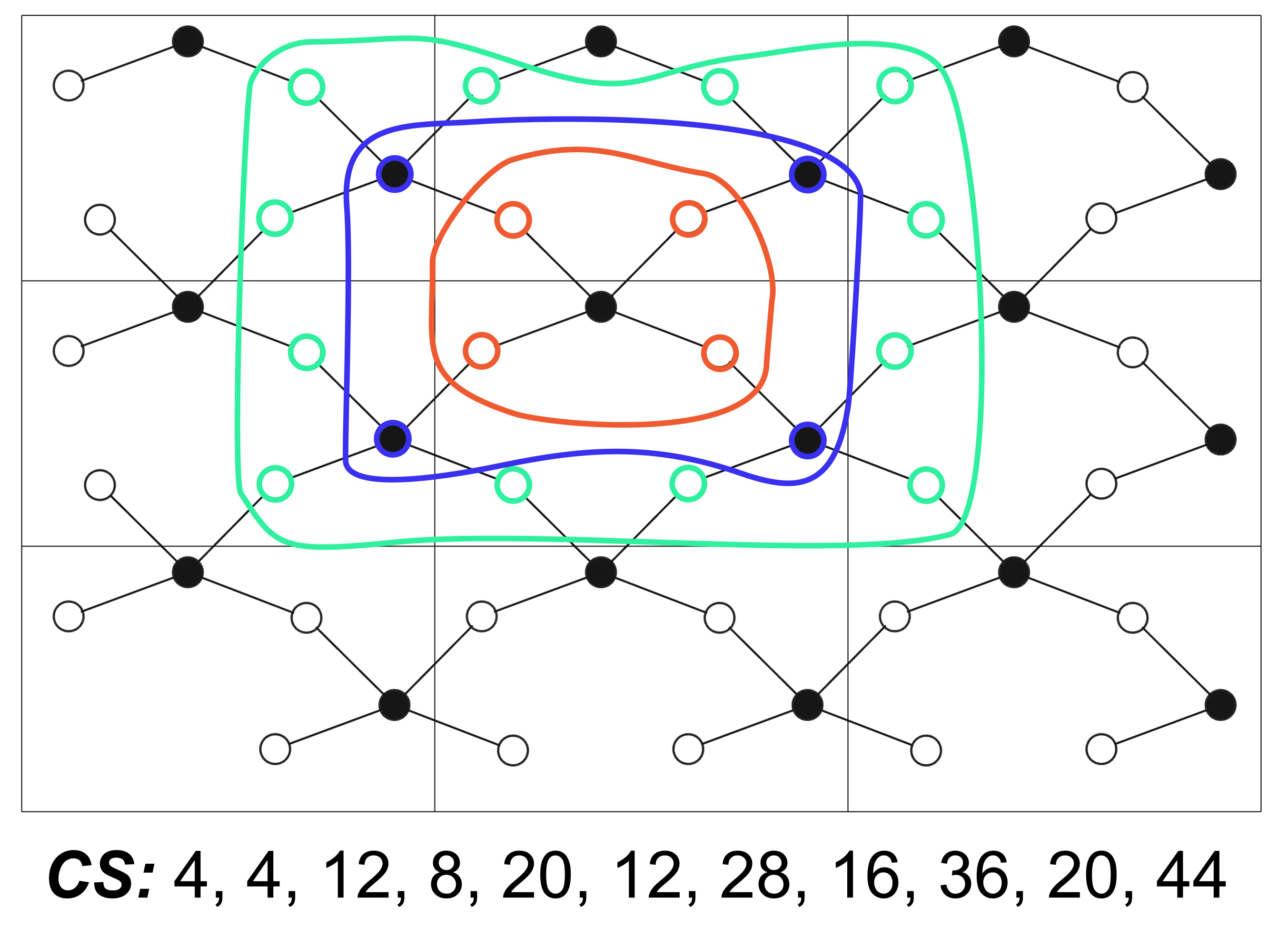
To compare the graphs, a method was used based on the calculation of coordination sequences: the number of vertices in the first, second, third, etc. vertex coordination spheres. Having calculated the coordination sequences of all vertices of the factorgraph, one can find topologically equivalent vertices. The coincidence of such a set of sequences for two graphs is a necessary but not sufficient condition for their identity. For example, the coordination sequences of two different lattices of RHO and LTA zeolites completely coincide. In such cases, one resorts to the analysis of additional parameters (for example, vertex symbols). The program was written in python using the ase, spglib, pyxtal and networkx libraries. Website design created using Nicepage.
Refs:
Structural Crystallography of Inorganic Oxysalts Krivovichev, Sergey V. Oxford University Press, 2009. — P. 328.
The atomic simulation environment - A Python library for working with atoms Ask Hjorth Larsen, Jens JØrgen Mortensen, Jakob Blomqvist et al. // Journal of Physics Condensed Matter — 2017
Crystal chemistry and structural complexity of natural and synthetic uranyl selenites Vladislav V. Gurzhiy, Ivan V. Kuporev, Vadim M. Kovrugin et al. // Crystals. — 2019. — Vol. 9, no. 12.